We are excited to share that our newest manuscript has been published in Gels! Congratulations to Sara for her first paper with the lab!!
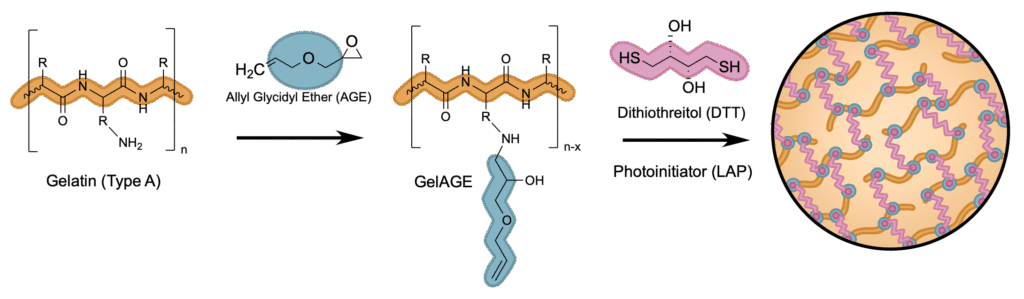
Our study investigates a novel thiol–ene based GelAGE hydrogel system designed to overcome key limitations of traditional covalently crosslinked gelatin hydrogels. By elucidating the molecular mechanism behind gelatin functionalization and crosslinking, we established a mechanistic framework for tunable, biocompatible 3D matrices that better mimic the viscoelastic and structural properties of cartilage. These insights pave the way for advanced in vitro models for osteoarthritis research and future applications in tissue engineering.
Abstract
Although covalently crosslinked gelatin hydrogels have been investigated for use in 3D cell culture due to inherent bioactivity and proliferation within the denatured collagen precursor, the stability of the matrix, and relatively inexpensive synthesis, current systems lack precise control over mechanical properties, including homogeneity, stiffness, and efficient diffusion of nutrients to embedded cells. Difficulties in modifying gel matrix composition and functionalization have limited the use of covalently crosslinked gelatin hydrogels as a three-dimensional (3D) cell culture medium, lacking the ability to tailor the microenvironment for specific cell types. In addition, the currently utilized chain-growth photopolymerization mechanism for crosslinking hydrogels has a potential for side reactions between the matrix backbone and components of the cell surface, requires a high concentration of radicals for initiation, and only cures with long irradiation times, which could lead to cytotoxicity. To overcome these limitations, a superfast curing reaction mechanism, in which a thiol monomer reacts efficiently with non-homopolymerizable alkenes, is suggested. This mechanism reliably produces a well-defined matrix that does not require a high radical concentration for photoinitiation. Mechanical customization of the hydrogel is largely achievable through variation in degree of functionalization of the gelatin backbone, dependent on reaction conditions such as pH, allyl concentration, and time. This work provides a mechanistic framework for GelAGE hydrogel fabrication by elucidating the molecular mechanism of gelatin functionalization with AGE and the thiol-ene crosslinking reactions controlling network stiffness. These insights provide the foundation for engineering hydrogels that mimic the viscoelastic and structural characteristics of cartilage, enabling advanced in vitro models for osteoarthritis research.
Find the full paper here:
S. Swank, P. VanNatta, M. Ecker, Elucidating the Chemistry Behind Thiol-Clickable GelAGE Hydrogels for 3D Culture Applications. Gels, 2025, 11(11), 874. https://doi.org/10.3390/gels11110874
